
About Us
Wellcare-Eurofins Collaboration
Wellcare Laboratories Ltd and EUROFINS have been in collaboration since 2020, services have been provided in our laboratory on the behalf of EUROFINS in the TRNC. With this collaboration, we ensure that the international services of EUROFINS company are brought to the highest level within the framework of scientific and ethical values in the country, and that we provide reliable and respected service in our laboratories by applying the most advanced technology standards.
Eurofins is the world's largest laboratory chain operating with 900 laboratories and 60,000 employees. Eurofins, which is structured in 4 main areas important for human health as Food, Environment, Biotechnology and Clinical Diagnosis, has put more than 7000 tests into service for human health.
Eurofins, which has 3 fully equipped and state-of-the-art Food and Environment Laboratories in Turkey; In 2018, decided to restructure themselves becoming 'Clinical Diagnostic' . With a team of 23 people in total, they continue activities in the field of NIPT, Oncology, IVF and External Lab tests (Sent-Out).
Eurofins, which uses the Illumina device, patented by NGS technology as NIPT, works with the latest version (Version 2 kits) and a fully automated system. All of our tests have CE-IVD approval; All of our laboratories are accredited and have the necessary quality control systems, documents and certifications.
Within the scope of NIPT Test Services offered in partnership with Wellcare Laboratories are:
• Genetic Counseling Service is provided by our 3 geneticists to our physicians upon their requests.
• For positive reports, analysis of diagnostic tests is offered as a free service to verify the test report.
• For more detailed information, the websites are as follows:
• www.eurofins.com/clinical-diagnostics/
• www.nipt-biomnis.com
• www.lifecodexx.com
• www.laboratoriogenoma.eu
NON INVASIVE PRENATAL TESTING (NIPT)
During pregnancy, parts of fetal DNA pass into the maternal blood and can be detected from the 7th week of pregnancy. The amount of circulating fetal DNA increases with increasing duration of pregnancy and is sufficient to guarantee a highly specific and sensitive NIPT screening result after the 10th week of pregnancy. Through the blood taken from the mother, it can detect changes in the genetic structure of the unborn child, that is, chromosomal disorders.
This test is a safe examination method for the unborn child. PrenatalSafe® can help relieve your worries and fears about certain health problems in the baby. Our NIPT tests have clinical performance data on millions of pregnant women published in clinical journals. With comprehensive genetic information, a broad screening test by analysis of cell-free fetal DNA (cffDNA) circulating in maternal blood includes the following numerical and/or structural anomalies, microdeletions, de-novo mutations, fetal RH incompatibility, genetic disorders at the single gene level in the fetus, and more.
Chromosomal Aneuploidies: They are chromosomal abnormalities characterized by changes in the number of chromosomes, i.e. more or less than the standard number of chromosomes. For example, we speak of trisomy when there is an extra chromosome or monosomy when there is a loss of a chromosome. Chromosomal trisomy aneuploidies in the test fetus; for example Down Syndrome, Edwards Syndrome, Pateu Syndrome, Monosomy X, Trisomy X, Klinefelter Syndrome, and Jacobs Syndrome, in addition it screens for all chromosomal monosomies and trisomies
TRISOMY 21 It is caused by the presence of an extra copy of the chromosome and is also known as Down Syndrome. It is the most common genetic cause of mental retardation. Trisomy 21 is estimated to affect about 1 in 700 newborns.
TRISOMY 18 It is caused by the presence of an extra copy of chromosome 18. Edwards syndrome is associated with a high abortion rate. It causes severe mental retardation. Babies with trisomy 18 often have congenital heart defects and other pathological conditions that shorten their life expectancy. Trisomy 18 is estimated to be present in 1/5,000 births.
TRISOMY 13 It is caused by the presence of an extra copy of the 13th chromosome. Also known as Patau Syndrome, it is highly associated with abortion. Babies with trisomy 13 have multiple heart defects, severe cognitive impairment, and developmental disabilities. They do not survive beyond the first months of life. It is a much less common condition than Down syndrome, which occurs in about 16,000 newborns. Structural Chromosomal Anomalies:The test examines structural anomalies as well as numerical anomalies in the fetal genome, providing information about both karyotype-level evaluation as well as insertions (duplication) and deletions (deletion) in chromosomes across the genome.
Microdeletion syndromes: Provides the opportunity to scan for clinically important microdeletions.
Fetal RH Incompatibility Test: The RhSafe ® test is a non-invasive prenatal test that consists of genotyping fetal RHD from maternal plasma of RhD - women, detecting the presence or deletion of the RHD gene (fetal RhD - ) or RHD gene (fetal RhD) in plasma. PrenatalSafe ® can be combined with the RhSafe ® test, a non-invasive prenatal examination that allows to determine the Fetal Rh(D) factor by analyzing fetal DNA isolated from a pregnant woman's blood sample. The RhSafe ® test is optional and is done on request in Rh(D) negative pregnant women with a Rh(D) positive partner. The RhSafe ® test can identify when immunoprophylaxis is unnecessary and can be avoided in pregnant women: Pregnant Rh- and Fetus Rh-.
PrenatalSafe ® & GeneSafeTM
• PrenatalSafe ® is offered by Eurofins Genoma Group laboratories with the option to choose from six levels of prenatal screening, each with a different level of detail. It is the most technologically advanced and most comprehensive Non-Invasive Prenatal Screening Test currently available. Today, it has become possible to screen other chromosomal anomalies at the karyotype level with PrenatalSafe® Karyo (autosomal anomalies, all deletions and duplications above 7 mb). Available non-invasive prenatal tests screen for aneuploidies and microdeletions. PrenatalSafe® Karyo also provides karyotype-level information by scanning for rare aneuploidies and segmental chromosome imbalances (gains and losses) on each chromosome in the fetal genome.
• GeneSafe™ goes even further, through cfDNA analysis from maternal plasma it screens several clinically significant and life-changing genetic disorders that are not screened by current NIPT technology.
This test is a safe examination method for the unborn child. PrenatalSafe® can help relieve your worries and fears about certain health problems in the baby. Our NIPT tests have clinical performance data on millions of pregnant women published in clinical journals. With comprehensive genetic information, a broad screening test by analysis of cell-free fetal DNA (cffDNA) circulating in maternal blood includes the following numerical and/or structural anomalies, microdeletions, de-novo mutations, fetal RH incompatibility, genetic disorders at the single gene level in the fetus, and more.
What does NIPT tests screen for?
Chromosomal Aneuploidies: They are chromosomal abnormalities characterized by changes in the number of chromosomes, i.e. more or less than the standard number of chromosomes. For example, we speak of trisomy when there is an extra chromosome or monosomy when there is a loss of a chromosome. Chromosomal trisomy aneuploidies in the test fetus; for example Down Syndrome, Edwards Syndrome, Pateu Syndrome, Monosomy X, Trisomy X, Klinefelter Syndrome, and Jacobs Syndrome, in addition it screens for all chromosomal monosomies and trisomies
TRISOMY 21 It is caused by the presence of an extra copy of the chromosome and is also known as Down Syndrome. It is the most common genetic cause of mental retardation. Trisomy 21 is estimated to affect about 1 in 700 newborns.
TRISOMY 18 It is caused by the presence of an extra copy of chromosome 18. Edwards syndrome is associated with a high abortion rate. It causes severe mental retardation. Babies with trisomy 18 often have congenital heart defects and other pathological conditions that shorten their life expectancy. Trisomy 18 is estimated to be present in 1/5,000 births.
TRISOMY 13 It is caused by the presence of an extra copy of the 13th chromosome. Also known as Patau Syndrome, it is highly associated with abortion. Babies with trisomy 13 have multiple heart defects, severe cognitive impairment, and developmental disabilities. They do not survive beyond the first months of life. It is a much less common condition than Down syndrome, which occurs in about 16,000 newborns. Structural Chromosomal Anomalies:The test examines structural anomalies as well as numerical anomalies in the fetal genome, providing information about both karyotype-level evaluation as well as insertions (duplication) and deletions (deletion) in chromosomes across the genome.
Microdeletion syndromes: Provides the opportunity to scan for clinically important microdeletions.
Fetal RH Incompatibility Test: The RhSafe ® test is a non-invasive prenatal test that consists of genotyping fetal RHD from maternal plasma of RhD - women, detecting the presence or deletion of the RHD gene (fetal RhD - ) or RHD gene (fetal RhD) in plasma. PrenatalSafe ® can be combined with the RhSafe ® test, a non-invasive prenatal examination that allows to determine the Fetal Rh(D) factor by analyzing fetal DNA isolated from a pregnant woman's blood sample. The RhSafe ® test is optional and is done on request in Rh(D) negative pregnant women with a Rh(D) positive partner. The RhSafe ® test can identify when immunoprophylaxis is unnecessary and can be avoided in pregnant women: Pregnant Rh- and Fetus Rh-.
PrenatalSafe ® & GeneSafeTM
• PrenatalSafe ® is offered by Eurofins Genoma Group laboratories with the option to choose from six levels of prenatal screening, each with a different level of detail. It is the most technologically advanced and most comprehensive Non-Invasive Prenatal Screening Test currently available. Today, it has become possible to screen other chromosomal anomalies at the karyotype level with PrenatalSafe® Karyo (autosomal anomalies, all deletions and duplications above 7 mb). Available non-invasive prenatal tests screen for aneuploidies and microdeletions. PrenatalSafe® Karyo also provides karyotype-level information by scanning for rare aneuploidies and segmental chromosome imbalances (gains and losses) on each chromosome in the fetal genome.
• GeneSafe™ goes even further, through cfDNA analysis from maternal plasma it screens several clinically significant and life-changing genetic disorders that are not screened by current NIPT technology.
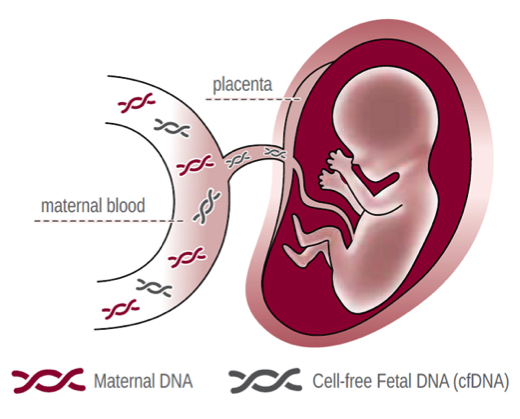
The amount of circulating fetal DNA from 9-10 weeks of pregnancy is sufficient to guarantee high specificity and sensitivity of the test.
The test is performed starting from a simple blood sample taken from the mother whose gestational age is at least 10 weeks.
Through laboratory analysis, free-circulating fetal DNA is isolated from the plasma component of maternal blood.
The next step is an advanced technological process, chromosomal regions of circulating fetal DNA are sequenced at a high read depth (~30 million sequences) of the entire fetal genome through innovative dense parallel sequencing (MPS) technology, using Illumina market-leading Next Generation Sequencing (NGS) technology.
Chromosomal sequences are finally measured through a bioinformatics analysis to determine the presence of any fetal chromosomal aneuploidies. Test failure rates are the lowest (0.3%).
The test is performed starting from a simple blood sample taken from the mother whose gestational age is at least 10 weeks.
Through laboratory analysis, free-circulating fetal DNA is isolated from the plasma component of maternal blood.
The next step is an advanced technological process, chromosomal regions of circulating fetal DNA are sequenced at a high read depth (~30 million sequences) of the entire fetal genome through innovative dense parallel sequencing (MPS) technology, using Illumina market-leading Next Generation Sequencing (NGS) technology.
Chromosomal sequences are finally measured through a bioinformatics analysis to determine the presence of any fetal chromosomal aneuploidies. Test failure rates are the lowest (0.3%).
OUR TEST OPTIONS
Our wide portfolio can help you choose the most suitable screening test is as followed: Always consult your doctor before deciding which test is right for you.

Screens for aneuploidies of chromosomes 21, 18, 13 and includes determination of fetal sex (optional).

Screens for aneuploidies of chromosomes 21, 18, 13, sex chromosomes (X and Y) and includes determination of fetal sex. (optional).

+ DiGeorge
Screens for aneuploidies of chromosomes 21, 18, 13, sex chromosomes (X and Y) and includes determination of fetal sex. (optional). Scans the trisomy of chromosomes 9 and 16. Detects the presence of microdeletion syndrome: 22Q11.2- DIGEORGE SYNDROME
Screens for aneuploidies of chromosomes 21, 18, 13 and sex chromosomes, as well as trisomy of chromosomes 9 and 16. The test also detects the presence of the 6 most common microdeletion syndromes. (22Q11.2 SYNDROME (DIGEORGE SYNDROME, VELOCARDIALFACIAL) 1P36 DELESION SYNDROME ANGELMAN'S SYNDROME (15Q11.2) PRADER-WILLI SYNDROME (15Q11.2) CRI DU CHAT SYNDROME (5P) WOLF-HIRSCHHORN SYNDROME (4P 9' and 16K)

It screens for aneuploidies and structural chromosomal changes (segmental deletions and duplications) of each chromosome

Screens numerical and structural anomalies in all chromosomes and 9 microdeletions; 22Q11.2 SYNDROME (DIGEORGE SYNDROME, VELOCARDIALFACIAL) 1P36 DELESION SYNDROME ANGELMAN'S SYNDROME (15Q11.2) PRADER-WILLI SYNDROME (15Q11.2) CRI DU CHAT SYNDROME (5P) WOLF-HIRSCHHORN SYNDROMUE (11Q11. 3 DELETION) LANGER-GIEDION SYNDROME (8Q24.11-Q24.13 DELETION) SMİTH-MAGENIS SYNDROME (17P11.2 DELETION)

It is the first non-invasive prenatal test to screen for both de-novo and inherited single gene disorders.
25 Gene Screening: Skeleton dysplasia, congenital heart defect, multiple congenital malformation syndromes, autism, epilepsy, mental disability, such as neurodevelopment disorders such as Schinzel-Gedion syndrome and Bohring-opitz syndrome, such as rare autosomal dominant mendelian disorders such as de novo mutation disorders. Performs screening of severe genetic diseases caused by gene mutations.
25 Gene Screening: Skeleton dysplasia, congenital heart defect, multiple congenital malformation syndromes, autism, epilepsy, mental disability, such as neurodevelopment disorders such as Schinzel-Gedion syndrome and Bohring-opitz syndrome, such as rare autosomal dominant mendelian disorders such as de novo mutation disorders. Performs screening of severe genetic diseases caused by gene mutations.

It is the first non-invasive prenatal test to screen for both de-novo and inherited single gene disorders.
5 Recessive Disease Screening
- CYSTIC FIBROSIS; GEN CFTR
- AUTOSOMAL RECESSIVE HEARING LOSS- TYPE1A; GEN CX26 (GJB2)
- AUTOSOMAL RECESSIVE HEARING LOSS- TYPE1B; GEN CX30 (GJB6)
- BETA thalassemia; GENE HBB
- SICKLE CELL ANEMIA; GENE HBB
5 Recessive Disease Screening
- CYSTIC FIBROSIS; GEN CFTR
- AUTOSOMAL RECESSIVE HEARING LOSS- TYPE1A; GEN CX26 (GJB2)
- AUTOSOMAL RECESSIVE HEARING LOSS- TYPE1B; GEN CX30 (GJB6)
- BETA thalassemia; GENE HBB
- SICKLE CELL ANEMIA; GENE HBB

GENESAFE DE NOVO + GENESAFE INHERITED

NIPT KARYO + GENESAFE COMPLETE

NIPT KARYO PLUS + GENESAFE COMPLETE
The purpose of our NIPT screening test is for patients who meet any of the following criteria:
- Both singleton and twin pregnancies
- Pregnancies achieved by IVF techniques, including egg donation or surrogacy
- Abnormal ultrasound findings
- Parents at risk for screened genetic conditions
- Advanced maternal age (over 35 years) and paternal age (over 40 years)
- Positive results on maternal serum screening
- Patients wishing to avoid the invasive diagnostic procedure
Why Choose PrenatalSAFE?
ADVANTAGES OF THE PRENATALSAFE TESTS
PrenatalSafe® detects conditions that other tests fail to detect, including rare trisomies, segmental chromosomal abnormalities, and monogenic disorders (hereditary and de novo).
• PrenatalSafe® has the lowest false-negative rate: 0% in published clinical studies.
• PrenatalSafe® distinguishes between maternal and fetal DNA, which helps prevent false positives.
• Of all NIPTs available on the market, PrenatalSafe® has the highest published performance.
• PrenatalSafe® can detect chromosomal abnormalities in the low fetal fraction (<4%).
• The PrenatalSafe® test offers a depth of resolution (>60 Million readings) unlike any non-invasive prenatal test available to date.
• PrenatalSafe® test, in an ISO 17025 approved laboratory with more than 20 years of experience in prenatal diagnosis, more than 200,000 genetic tests are conducted annually.
• The PrenatalSafe® test is recommended by a large number of gynecologists worldwide.
• The PrenatalSafe® test is the only NIPT to date to screen for both genome-wide chromosomal abnormalities and serious genetic disorders in the fetus in a single test.
• Unlike other NIPTs available on the market, the Prenatalsafe® test provides a free Rh(D) test (RhSafe). (In case of Rh(D) negative mother and Rh(D) positive father)
• The PrenatalSafe® test is suitable for both singleton and twin pregnancies (including lost twins), as well as pregnancies obtained through IVF techniques, including gamete donation.
• PrenatalSafe® also provides genetic counseling in case of positive results.
• PrenatalSafe® testing is done in Italy (Rome or Milan)
SAFE AND RELIABLE
• It is a non-invasive test and poses no risk to the fetus or mother. It is a screening test in which circulating fetal DNA is analyzed at 10+ gestational weeks.
• The PrenatalSafe ® test has a sensitivity and specificity of over 99% with a very low false positive rate in less than 0.1% of cases.
• PrenatalSafe ® Karyo Plus shows 99.1% of fetal chromosomal aneuploidies present at birth and achieves a detection rate very similar to conventional (96.9%) and molecular (99.8%) fetal karyotypes obtained by invasive prenatal diagnostic techniques.
• Comparison with Invasive Prenatal Diagnosis The PrenatalSafe® 3 and 5 test allows highlighting 71% and 83.1%, respectively, of chromosomal abnormalities found during pregnancy. With the prenatal SAFE® Plus test, the detection rate reaches 86%. The PrenatalSafe® Complete test represents the highest level of research available. The test allows to detect 95.5% of detectable chromosomal abnormalities in pregnancy (Karyo Plus research level), achieving a detection rate very similar to the traditional fetal karyotype (96.9%) obtained by invasive prenatal diagnostic techniques. It also has the ability to identify mutations responsible for serious genetic diseases.
SENSITIVE
• The Limit of Detection (LOD) of the test is determined in the 2% fetal fraction (FF). The value of the fetal fraction is always reported in reports.
PRE-TEST AND POST-TEST GENETIC CONSULTANCY
• To explain to patients the purpose of the analysis, the results that can be obtained, and what happens after the examination is complete.
SPEEDY PROCEDURE
• Thanks to the recent introduction of new high-resolution FAST Technology, results are available in just 3-5 working days after samples arrive at the lab.
RHSAFE® TEST
• Non-invasive prenatal examination that allows to determine the fetal Rh (D) factor. The RhSafe® test is optional and is done free of charge (on request) in Rh(D) negative pregnant women.
FREE TRACKING OF PATHOLOGICAL RESULTS
• In cases of positive pathological results with the presence of aneuploidies, structural chromosomal anomalies or genetic mutations, free follow-up is offered by Eurofins Genoma to the referring gynecologists.
SAMPLE COLLECTION AND TRANSPORTION KIT
• Certification according to UN3373 and free shipping of biological samples to our laboratories
ALL INCLUSIVE CUSTOMER SUPPORT
• Information on sample collection and storage methods, from sample shipping to reporting.
EXCLUSIVE AND SCIENTIFIC SUPPORT
• Qualified molecular biologists and geneticists are always available to assist our patients in interpreting the results.
Frequently Asked Questions
What is the PrenatalSafe ® screening test?
PrenatalSafe ® is the first prenatal screening test that can detect from the most common fetal aneuploidies (13, 18, 21, X and Y) to the rarest aneuploidies (all chromosomes of the fetal karyotype). PrenatalSafe ® is also the only non-invasive prenatal screening test that can highlight subcosomal structural abnormalities and mutations associated with serious genetic diseases across the entire fetal karyotype. The test also provides information about the sex of the fetus (optional).
Who can do the PrenatalSafe® Non-invasive prenatal screening test?
PrenatalSafe® can be performed by all pregnant women whose gestational age is at least 10 weeks. PrenatalSafe ® can be applied in single pregnancies, both homologous and heterologous pregnancies obtained by in vitro fertilization (IVF, ICSI), twin pregnancies even if obtained with assisted, homologous and heterologous fertilization techniques.
PrenatalSafe ® Complete and PrenatalSafe ® COMPLETE PLUS are especially suitable for couples of advanced paternal age.
When can the PrenatalSafe® screening test be performed?
PrenatalSafe® is available from the 10th week of pregnancy. There is no deadline for performing the test because fetal DNA remains in the mother's bloodstream throughout pregnancy.
How accurate is the PrenatalSafe® screening test?
The PrenatalSafe ® screening test has a sensitivity and specificity of greater than 99% with a very low incidence of false positives below 0.1%. Prenatal SAFE ® detects abnormalities affecting the fetal genome, even in low amounts of fetal DNA (FF≥ 2%).
What kind of information is reported in PrenatalSafe® screening test reports?
The results of the test are presented as POSITIVE or NEGATIVE, ie in clear and defined terms such as the presence/absence of the anomaly within the limits of the method used.
Is the PrenatalSafe ® screening test similar to other first and second trimester screening tests?
No . Screening tests are indirect statistical tests based on ultrasound examinations on the fetus and/or biochemical examinations in maternal blood, in which some substances, the amounts of which can vary if there are some chromosomal pathologies, are measured. Prenatal SAFE ® test, as well as a screening test, is a direct analysis of fetal DNA and analyzes fetal DNA circulating in maternal blood with great accuracy.
Is PrenatalSafe ® more reliable than currently available non-invasive prenatal screening tests?
Prenatal screening tests such as the bi-test may pose a high risk for fetal trisomy or present a low risk for fetal trisomy, even if there is actually a negative (false-positive) result, when in reality it is a positive pregnancy (false-negative). These non-invasive tests, such as the combination of free-b-HCG and PAPP-A proteins with nuchal translucency, have a false-positive rate of up to 5% and do not detect approximately 10-15% of fetal trisomy 21 cases. With the PrenatalSafe ® screening test, the false positive and negative percentages are reduced to 0.1%.
Is PrenatalSafe ® a safe test or does it involve risks?
The PrenatalSafe ® screening test is completely safe for both mother and fetus . Although diagnostic tests such as amniocentesis or CVS are accurate for diagnosing fetal trisomies, they are invasive and carry a non-negligible risk of miscarriage and require appropriate antibiotic therapy.
Some Test Procedures in Our Hospital
Would you like to visit our hospital?
Make an appointment now!

Welcome to our hospital
Medical Port Tunççevik Hospital is an institutional, güler-faced and fully equipped health center that targets the healthy life of you and your family and includes all branches.
Policlinic Working Hours
- 800 - 1800
- 800 - 1400
Our hospital has 7/24 fully equipped Emergency Service Support
Contact Us
- Kurtuluş Caddesi,
Numara 70, Bellapais Yolu,
Girne, KKTC
(Altınkaya II Yanı)
